- home Home
- keyboard_arrow_right Uncategorized
- keyboard_arrow_right Posts
- keyboard_arrow_rightCOVID-19 vaccine: the challenges of running a trial in the middle of a pandemic
COVID-19 vaccine: the challenges of running a trial in the middle of a pandemic
JBy: Jeffrey Mphahlele, South African Medical Research Council
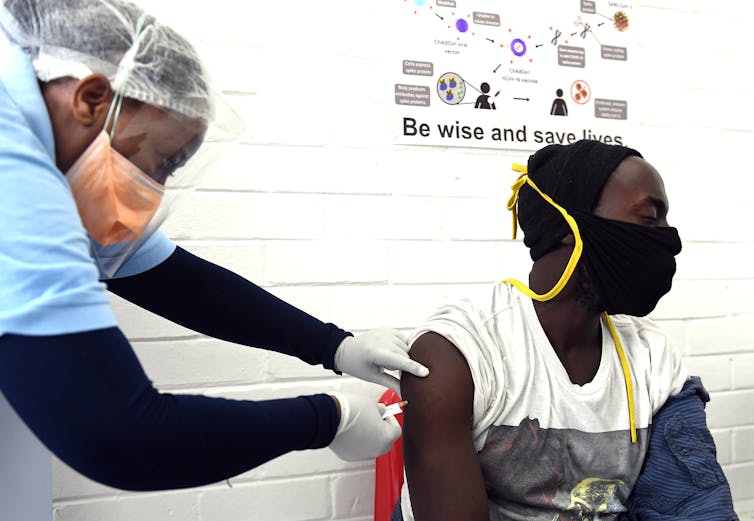
Felix Dlangamandla/Beeld/Gallo Images via Getty Images
South Africa recently announced the start of the country’s first COVID-19 vaccine clinical trial. The vaccine is also being tested in the UK and Brazil. Ina Skosana spoke to the vice-president for research at the South African Medical Research Council, Professor Jeffrey Mphahlele, to find out more.
How big is the trial and who’s involved?
The trial is a joint effort between different stakeholders. The main player is the University of the Witwatersrand, under the leadership of Professor Shabir Madhi. The South African Medical Research Council is co-funding the project with R10 million. Other funders include the Bill and Melinda Gates Foundation and vaccine developers at the University of Oxford.
In South Africa the aim is to vaccinate around 2000 volunteers. This will happen in at least three different groups. Half of the participants will receive the experimental vaccine and half will receive placebo. They’ll be followed for about a year to assess the safety of and the immune response to the experimental vaccine.
Before enrolling the large group of volunteers, the trial will start with a group of about 50 HIV negative healthy volunteers to evaluate the vaccine safety and to a limited extend immune response. Later, the trial aims to investigate the vaccine safety and ability to mount the immune response in HIV positive volunteers. It’s important to test the vaccine in a participants with HIV given the high burden of HIV and AIDS in South Africa and the continent.
It’s prudent to evaluate the safety of the vaccine in this group during the clinical trial and not to wait until the vaccine is licensed. Even so, the vaccine, once licensed, will be for everybody.
At this stage, this particular vaccine candidate (ChAdOx1) is being tested in South Africa, Brazil and the UK.
If and when the vaccine is licensed, the aim is to make it a public good and available to all countries in the world – rich and poor.
What is the significance of Africa’s involvement?
It’s critical that developing countries are involved in clinical trials because it’s important to test the vaccine in different populations.
If the vaccine candidate is developed and tested in high income countries only, the efficacy may not necessarily be the same in populations living in low- and middle-income countries.
This has happened with vaccines before.
One example was the rotavirus vaccine candidates. The purpose of the rotavirus vaccine is to protect against dehydration and severe gastroenteritis. Early vaccine candidates demonstrated high efficacy of over 80% when tested in developed countries with low child mortality. But when the same vaccines were tested in low-income and middle-income countries of Africa and Asia, the efficacy was between 40% and 65%.
That did not rule out the use of rotavirus vaccines in the developing world. In fact, research has now shown that there has been a significant reduction in hospital or emergency unit admissions for acute rotavirus gastroenteritis, or mortality in children under five years old, in countries with rotavirus vaccination programmes. This was particularly true for low-income and middle-income countries with high child mortality.
There are a number of reasons why vaccine efficacy differs. These include the genetic background of the population. With the rotavirus vaccine, additional factors that are thought to affect optimal vaccine efficacy may include early age of first infection before administration of the first vaccine dose, maternal antibodies, distinct medical problems (high levels of HIV, TB, malaria, high background of enteric infections and malnutrition), and unique diversity of circulating rotavirus strains not included in the vaccines.
In the case of COVID-19 the virus seems to be genetically stable. This is a big plus.
What challenges does this trial present?
The most important challenge is the fact that a clinical trial is being conducted in the middle of a pandemic. It means that countries are having to deal with managing a public health emergency while simultaneously setting aside resources to do research.
Public health emergencies demand a response programme that is effective and nimble. No doubt the number one priority is to save lives and contain the epidemic. The recent Ebola outbreak in West Africa was a game changer in many ways. The outbreak spiralled out of control to become a global public health emergency due to a number of factors ranging from poor health systems to lack of medical innovations. When the cause of public health emergencies is known or predictable, and the tools to respond and intervene are widely available, it is always easy to respond and save lives.
We have learned a great deal since Ebola and other outbreaks. A key lesson is that research should take centre stage and become the norm in responding to a public health emergency – especially when the cause is unknown or novel – like with SARS-CoV-2. Rapid and responsive research during a public health emergency should aim to optimise and field test development of new health interventions such as vaccines, therapeutics and rapid diagnostic tools. It should also include socio-behavioural research, medical anthropology research and applied and translational research.
Read more:
Ebola in the DRC: the race is on between research and the virus
In the case of the COVID-19 vaccine trial in South Africa, rising numbers of infections create a conducive environment to conduct the trial because high levels of infections are needed to field test the vaccine efficacy. Doing this clinical trial at a time of low infections would take longer to yield such results. But now that the cases are spiking, we should be able to know sooner if the vaccine is likely to work or not.
As indicated above, this is not the first time research is conducted in the middle of an outbreak. We’ve got experience from Ebola, and other pathogens that were associated with outbreaks and epidemics like the original SARS, the MERS coronavirus and the Zika virus. These viruses were associated with epidemics – obviously not to the same magnitude as SARS-CoV-2.
Read more:
Mutating coronavirus: what it means for all of us
We just need to keep a balance between saving lives and doing research. In a pandemic like this one, the priority is to save lives. But there’s a limit in what we can use to save lives unless research innovation takes centre stage. Initially, the focus was on diagnostic tests. This has now shifted to treatment and the prevention regimens.
We’re making strides in treatment and prevention strategies. Recent breakthroughs include the use of remdesivir from the US and dexamethasone from UK for COVID-19 related treatment.
How is this trial similar to others?
Clinical trials are standardised so I don’t see this trial being different from others. Researchers should conduct trials following established legal, ethical and regulatory procedures governing conduct of trials. These include, but are not limited to, obtaining clearance from the South African Health Products Regulatory Authority and institutional Human Research Ethics Committees.![]()
Jeffrey Mphahlele, Vice President for Research, South African Medical Research Council
This article is republished from The Conversation under a Creative Commons license. Read the original article.
Written by: Natasha
Similar posts
MORE ARTICLES

‘You are my heart in human form’: Lungile Thabethe’s touching letter to her daughter

Smart tax moves: A guide to boosting your tax refund

‘My husband won’t back me up when I discipline his kids’ – The Blind Spot

Kai Cenat graces the cover of Time Magazine’s first-ever TIME100 Creators List

Tebogo Thobejane pens emotional tribute to son on his 18th birthday
QUICK LINKS
UpComing Shows

959 Music Weekdays
Kaya 959 Hits
Real. Familiar. Memorable. Kaya 959 brings you the music you know and love from our playlist. Uninterrupted. Thursdays 20h00 to 21h00
close
The Best T in the City
With T Bose
He has held it down in the world of mid-morning radio with the best music, riveting topics, brilliant mixes and interesting guests. Every weekday, The Best T proves why he is the BEST by connecting to you like only your bro or favourite uncle could. He lets his listeners dictate the songs they want to hear in the ever-popular Top 10 at 10, and his Three Teaspoons never run out. Catch The Best T in the City Mondays to Fridays from 09h00 to 12h00.
close
Feel Good
With Andy Maqondwana
Feel good about feeling good! That's exactly what The Feel-Good show is about. An escape from the negativity that surrounds us, indulging you in good feels. Pass it on to one and all. Spread the good feeling around Gauteng with Andy Maqondwana.
close
The Hive
With Bonolo "Bee Sting" Molosiwa
Every "Hive" needs a Queen B and Bonolo "Bee Sting" Molosiwa is Kaya 959's honey who brings in the money. With her bubbly personality, infectious laugh, Bee Sting radiates positive energy which is all you need to get your weekend off to the best start. Don't miss the Afrobeat Dancehall Ragga (ADR) Top 10 on The Hive with Bee Sting every Saturday from 18h00 - 21h00.
closeConnect with Kaya 959
DownLoad Our Mobile App
© 2025 Kaya 959 | On The Street On The Air










